Mobility_APP Study: Investigating the Efficacy of Gait-Specific Physiotherapy in Parkinson’s Disease
Parkinson’s disease (PD) can cause a variety of symptoms that require not only medication but also other interventions – such as physiotherapy. Physiotherapy is crucial in the care of Parkinson’s disease because it can help to improve mobility, balance and muscle strength – to maintain independence and satisfactory quality of life.
Mobility_APP is a novel clinical research study that focuses on physiotherapy intervention for individuals with Parkinson Syndromes (idiopathic Parkinson’s disease (PD) and atypical Parkinsonism such as Multiple System Atrophy (MSA) and Progressive Supranuclear Palsy (PSP). Conducted across multiple European sites, including Luxembourg, Austria, Germany, Switzerland, and Italy, the study is designed to contribute valuable insights into enhancing the mobility in people affected by these diseases.
More specifically, the study aims to assess and compare the benefits of two physiotherapy programs—gait-focused physiotherapy and general physiotherapy - for both people idiopathic Parkinson's and people with atypical Parkinsonism.
Timeline
The study is comprised of 2 main phases:
- Phase 1: In this intensive two-week period participants will have daily physiotherapy at physiotherapist’s (PT) office (1h a day for 2 weeks excluding weekends),
- Phase 2: Subsequently, participants will perform exercises at home individually for 5 weeks with remote supervision by physiotherapist (weekly phone calls).
The study follows a structured timeline over a period of 8 weeks total.
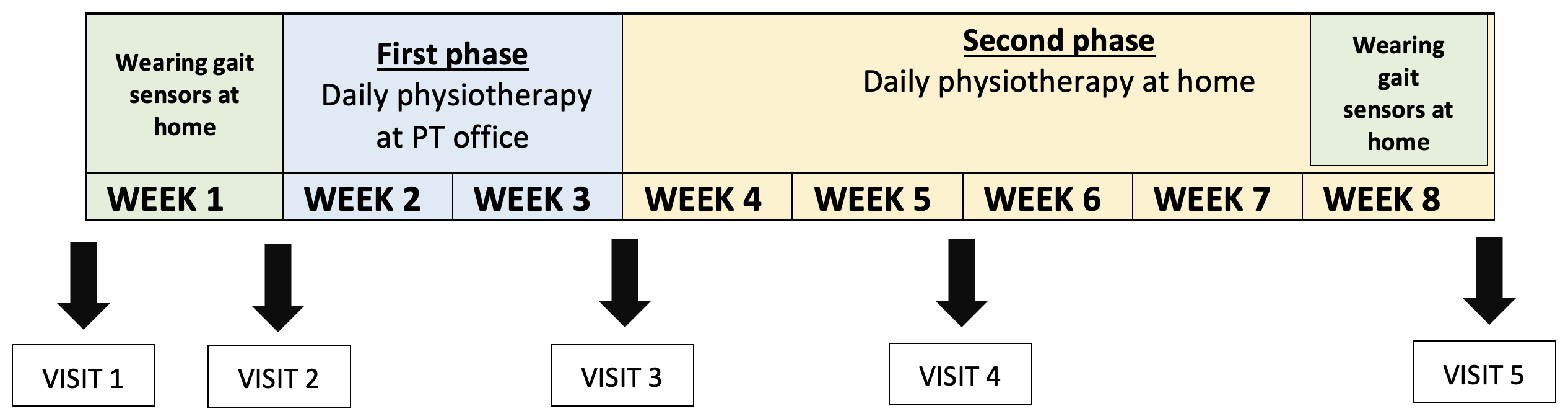
Fig. 1: Study timeline
Besides the physiotherapy described above, participants will have 5 visits to the Parkinson’s Research Clinic in this period. During these visits, several clinical assessments will be conducted including sensor-based gait analysis, clinical examinations (to evaluate mobility, cognition, non-motor symptoms etc.) and other relevant metrics to comprehensively evaluate the impact of the physiotherapy interventions.
Participants will also be given gait sensors (see Fig. 2) to wear at home for 1 week at the beginning and at the end of the study. They can easily be clipped to your regular shoes.

Fig. 2: Gait sensors
In Luxembourg, the study aims to recruit a total of 20 patients: 10 individuals with idiopathic Parkinson's and 10 with atypical Parkinsonism. Currently, we are actively recruiting participants.
We also kindly invite physiotherapists to join the study or refer your patients to our team. So far, four ParkinsonNet physiotherapists are already performing study-related physiotherapy programs and assisting in recruiting the patients.
By shedding light on the effectiveness of gait-specific physiotherapy in Parkinson's disease, the Mobility_APP study seeks to make meaningful contributions to the advancement of non-pharmacological interventions, ultimately improving the quality of life for individuals affected by Parkinsonism.
For additional information about the study or if you wish to participate personally/ refer your patient, please contact us at: marijus.giraitis [at] lih.lu / +352 621 574468
Data and samples
Your clinical data will be collected at the Parkinson’s Research Clinic in a pseudonymised way without any direct link to your personal information. Thus, none of your explicit identity, like your name or address, is included in or directly associated with the Mobility_App datasets. Therefore, your privacy is best preserved.
Your clinical information will be collected using questionnaires and physical assessments performed by clinical study team- we will evaluate various domains of PD - such as your motor and non-motor symptoms. Additional to clinical assessments, your gait and mobility will be evaluated using certified wearable gait sensors – we will clip these sensors to your shoes and ask you to do a series of walking exercises.
In all aspects of the data collection, we adhere strictly to the new European data protection regulations (GDPR). Your coded (pseudonymised) data will be analysed by dedicated members of the LCSB (Luxembourg Centre for Systems Biomedicine) and LIH (Luxembourg Institute of Health). Your data may also become available for future studies in anonymised form. The results of these studies will be likely published in medical and scientific journals. In this case, all results shall be published anonymously to ensure that you cannot be identified. Your name and identity will not appear in any of the published materials.
Detailed information on how we handle data and samples are described in the Study Information Sheet of the study.




